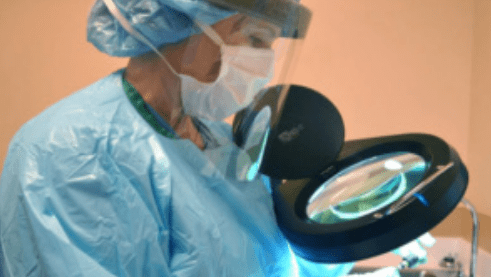
Customers often ask how they can implement cleaning verification as part of their Quality Control Process for reprocessing Flexible and Semi Rigid Endoscopes.
Sterile processing professionals are expected to verify that they are able to clean, disinfect, and sterilize instruments using automatic cleaning equipment and manual cleaning processes according to the manufacturer’s instructions for use (IFU).