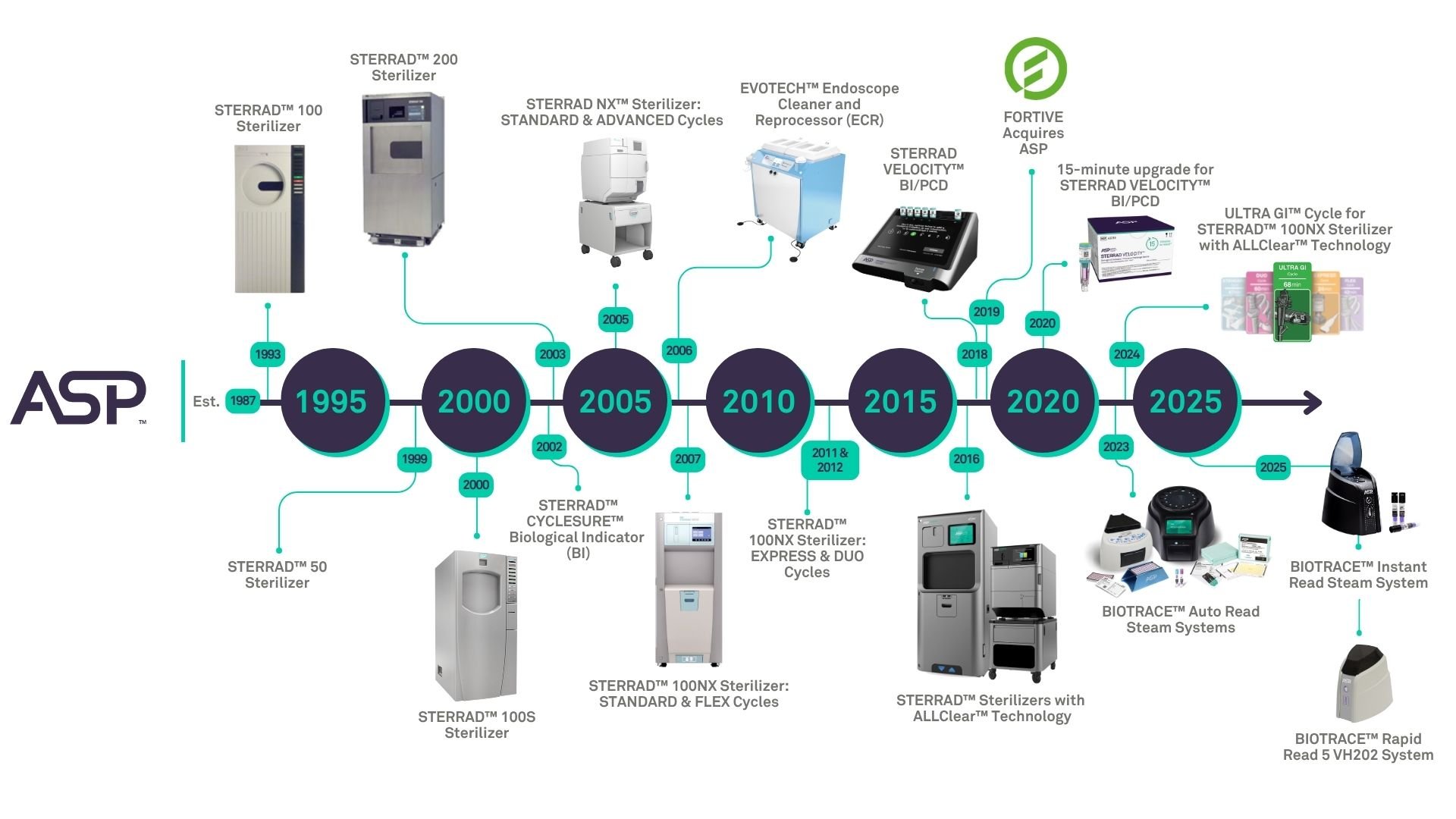
A group of scientists from a company in Texas pioneered the novel idea of using hydrogen peroxide for device sterilization. In 1987, this company officially formed as Advanced Sterilization Products.
ASP’s first vaporized hydrogen peroxide gas plasma sterilizer, the STERRAD™ 100 Sterilizer received FDA clearance in 1993. This first-of-its-kind technology sterilizes medical devices by diffusing hydrogen peroxide vapor into the chamber and then electromagnetically exciting the hydrogen peroxide molecules into a low-temperature plasma state. The combined use of hydrogen peroxide vapor and plasma safely and rapidly sterilizes medical instruments and materials without leaving toxic residue.
ASP continued to innovate over the years, introducing other sterilization systems such as the STERRAD NX™ Sterilizer in 2005 and STERRAD™ 100NX Sterilizer in 2007, both released with a Standard cycle for general medical instruments and an ADVANCED (STERRAD NX™ Sterilizer) and FLEX (STERRAD™ 100NX Sterilizer) cycle for single-channel flexible endoscopes. In 2006, the EVOTECH™ Endoscope Cleaner and Reprocessor (ECR), a premium, dual basin system that automates cleaning and high-level disinfection of endoscopes was launched.1 The STERRAD™ 100NX Sterilizer then received cycle upgrades including an EXPRESS Cycle, compatible with the Intuitive Surgical® da Vinci® Surgical Endoscope in 2011 and a DUO Cycle in 2012, validated for flexible scopes and cameras. ASP then introduced the ALLClear™ Technology software upgrade for the STERRAD NX™ Sterilizer and the STERRAD™ 100NX Sterilizer in 2016, which allowed for less canceled cycles, connectivity to the ASP ACCESS™ Data Station and hospital’s ITS, plus on-screen guides to increase compliance. In 2018, ASP introduced the STERRAD VELOCITY™ Biological Indicator (BI)/Process Challenge Device (PCD), providing a fast and reliable way to verify the effectiveness of the STERRAD™ Sterilization Cycle. The ASP AEROFLEX™ Automatic Endoscope Reprocessor (AER) was introduced in 2019 single basin system that automates the washing and high-level disinfection cycle of flexible semi-critical endoscopes.2 In 2020, the 15-minute BI software upgrade for STERRAD VELOCITY™ BI/PCD was released. In 2023, ASP expanded its sterilization monitoring portfolio with the introduction of new steam monitoring products, including the BIOTRACE™ Auto Read Steam Readers, BIOTRACE™ Biological Indicators, VERISURE™ Chemical Indicator Strips, and SEALSURE™ Steam Indicator Tape, broadening our biological and chemical monitoring solutions for steam sterilizers. In 2024, the ULTRA GI™ Cycle for the STERRAD™ 100NX Sterilizer with ALLClear™ Technology launched, the first and only FDA-cleared hydrogen peroxide gas plasma terminal sterilization method for duodenoscopes.5 In 2025, ASP continued to innovate with two major project launches, including the BIOTRACE™ Instant Read Steam Biological Indicator (BI) System, the fastest Steam BI on the market delivering results in only 7 seconds and the BIOTRACE™ Rapid Read 5 VH202 Biological Indicator System, the fastest VH2O2 biological indicator reader on the market, delivering actionable results in as little as 5 minutes.