Product Overview
Industry Standards and Guidelines Adherence
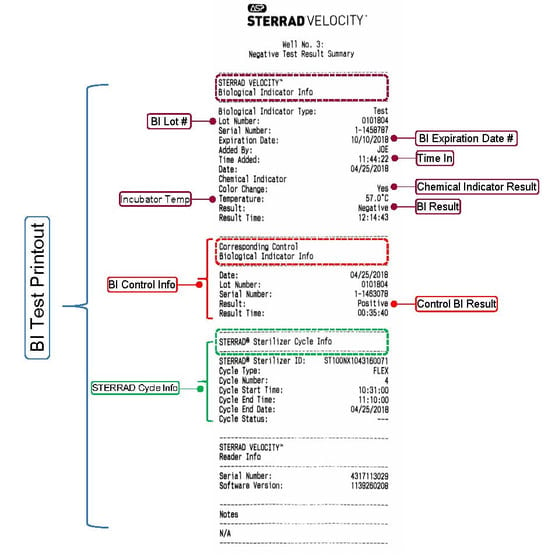
STERRAD VELOCITY™ Biological Indicator/ Process Challenge Device (PCD) is the first and only all-in-one used for H2O2 Sterilization.
- The only all-in-one BI/PCD that meets AAMI ST582 guidelines for every load monitoring (ST58 9.5.4.3).
- Accuracy that minimizes false positives and reduces time and cost of reprocessing.
IFU
The ASP IFU and User Guide library allows users to search by language, country and product to locate the appropriate instructions for use of ASP Products. Product literature and availability varies by country. Check out this helpful reference site to locate the STERRAD VELOCITY™ Biological Indicator / Process Challenge Device instructions for use.
115 or 30 minutes to results dependent on software version of the STERRAD VELOCITY™. Refer to the IFU for actual time to results. Fastest BI/PCD currently marketed for STERRAD™ Sterilization Systems
2ANSI/AAMI ST58 2013/(R)2018. Chemical Sterilization And High-Level Disinfection in Health Care Facilities (ST58).
3Requires ASP ACCESS™ Technology