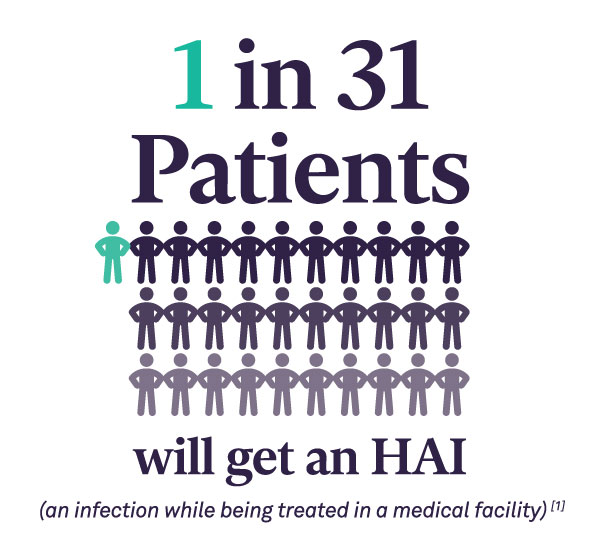
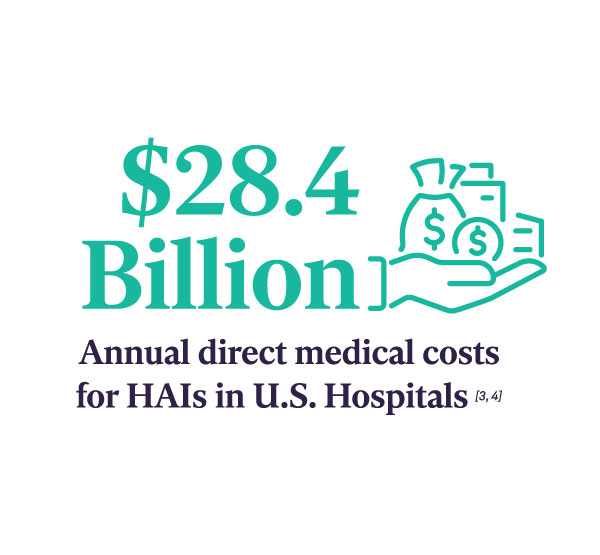
In recent years, The Joint Commission (TJC) is focusing on the Sterile Processing Department (SPD) as a priority area in surveys and found inconsistent adherence to processes. This has prompted the accrediting body to publish SPD-specific recommendations to improve patient safety.
This whitepaper from clinical experts at ASP and Virginia Commonwealth University details how to prepare the SPD for TJC surveys, avoid pitfalls, and implement best practices as demonstrated in a real-world environment.
According to recent findings, TJC reported that approximately half of the surveyed hospitals exhibited some degree of non-compliance2.