Introduction
Business resiliency refers to an organization's ability to adapt, recover, and thrive in the face of unexpected disruptions or challenges. It involves developing strategies, processes, and systems that enable a business to withstand and recover from various threats. Business resilience and the Sterile Processing Department (SPD) are not usually words one sees in the same sentence, but it’s time for hospital administration to start looking at the SPD through a different lens – one that reflects a department that contributes to revenue vs. purely a cost center to the operating room (OR). Business resilience encompasses more than continuity or disaster preparedness; it is the ability to respond, adapt, and keep the operation running in the event of any planned or unplanned challenge or disruption. This article looks at the important role the SPD plays in helping the OR and the facility to remain nimble and able to adapt to sudden changes and how proactive investments in equipment and staff can help ensure a culture of safety and a healthy revenue stream.
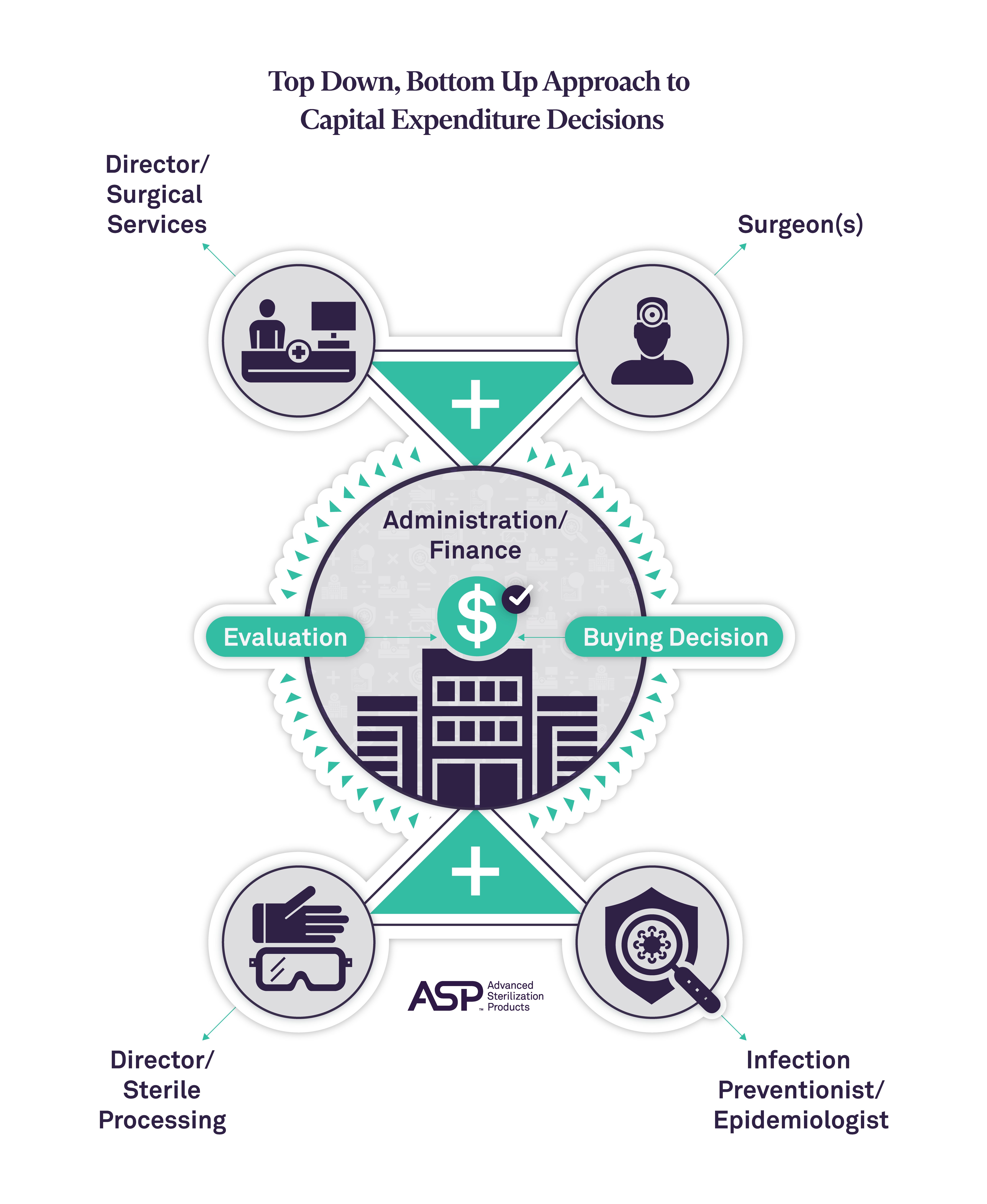
Applying Business Resilience to the SPD
The responsibility for cleaning, sterilizing, and maintaining medical devices and instruments lies with the SPD. Any deviation from normal operations, such as a poorly cleaned instrument reaching the operating room and causing harm to a patient, or a malfunctioning sterilization or endoscope reprocessing unit leading to the cancellation of a procedure, has a direct impact on patient safety, quality of care, and the financial stability of the healthcare facility. These can cause a case to be delayed or cancelled. In fact, The Joint Commission cited adherence to high-level disinfection and sterilization procedures for medical equipment and devices as one of the five areas noted for non-compliance during its recent survey.1
Business resilience in the SPD is imperative to ensure that there are no interruptions to patient care and safety. This encompasses maintaining compliance with regulations, policies, and procedures and contributing to overall facility performance. Four specific areas where business resilience is essential to the SPD include:
- Patient Safety: Effective business resilience policies play a crucial role in safeguarding patient well-being by mitigating interruptions and disturbances in the provision of sterile instruments and medical devices to the operating room. A resilient SPD should be adequately prepared and equipped to handle unforeseen events such as natural disasters, equipment malfunctions, or emergencies, all while ensuring a continuous supply of materials for the operating room. By guaranteeing the availability of sterile equipment, the likelihood of hospital-acquired infections (HAIs) or surgical site infections (SSIs) is significantly reduced, thereby enhancing patient safety and overall outcomes.2
- Regulatory Compliance: The SPD must adhere to strict regulations and standards as well as policies and procedures set forth by the facility and as directed by Infection Prevention. A business resilient SPD ensures full compliance by having the right processes, reliable equipment, and competent, well-trained staff in place.
- Operational Continuity: A well-prepared SPD that prioritizes business resilience implements various strategies and approaches such as thorough contingency and disaster preparedness plans, redundant systems for crucial operations, and a staff trained in multiple areas. This ensures minimal downtime and enables the department to swiftly recover and resume regular operations, thereby minimizing disruptions to the operating room, patients, surgeons, and the facility. By maintaining operational continuity, the department reduces delays and cancellations in the operating room, enhances patient safety, and mitigates financial losses for both the facility and surgeons.3
- Risk Management: What can possibly go wrong in the SPD? Plenty! SPD risks include equipment malfunctions and break down, equipment in need of replacement because it can no longer be repaired or has reached its end of life, HAIs or SSIs from medical devices and equipment that are not properly sterilized or reprocessed, staff shortages, and poorly trained staff. A risk averse SPD is one that proactively identifies and assesses risks, has a plan in place to mitigate or prevent risks, and collaborates with Infection Control to regularly review and update response plans. The SPD that effectively manages risk minimizes disruptions and ensures sterile instruments and medical devices are available when needed or requested to keep patients safe and the operating room running.