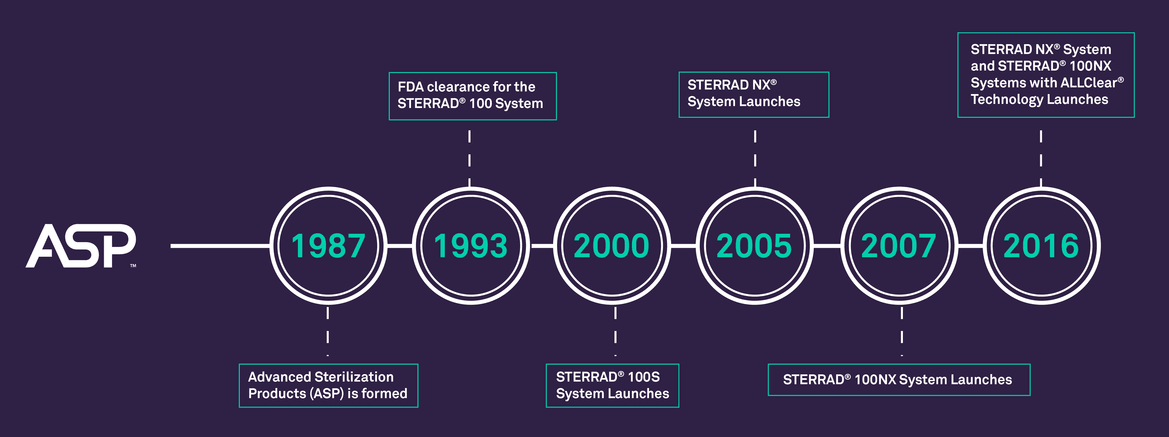
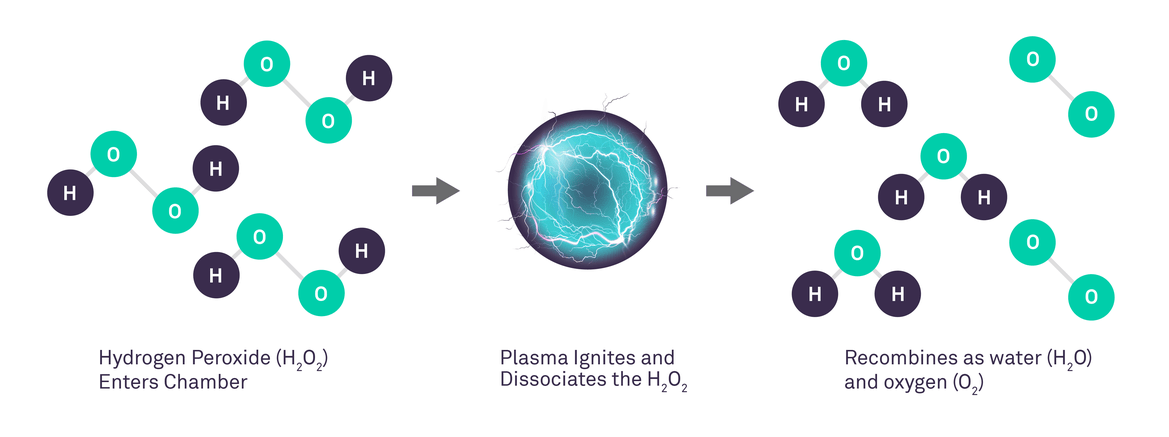
Vaporized Hydrogen Peroxide Gas Plasma Technology Explained
Advances in technology transformed many aspects of medicine such as robotic-assisted procedures in the modern world that lead to more complex surgical instruments. This posed challenges for sterilization using the traditional steam autoclave, and it paved the way for the development of other sterilization modality to process delicate instrumentation such as flexible endoscopes.
While Ethylene Oxide (EtO) was introduced as an alternative to steam sterilization for the sterilization of heat and moisture-sensitive instruments, there are many disadvantages associated with the use of EtO such as long sterilization cycles, cost and potential hazards to patient and staff.1