Introduction
The increased use of temperature-sensitive medical devices has given rise to higher usage of low temperature sterilization methods. STERRAD™ Systems and V-PRO® sterilizers offer shorter sterilization cycles and use hydrogen peroxide(H2O2), a sterilant with a better safety profile than ethylene oxide (EtO) sterilizers. Consequently, these sterilizers have become widely used for temperature- and moisture-sensitive instrument reprocessing.
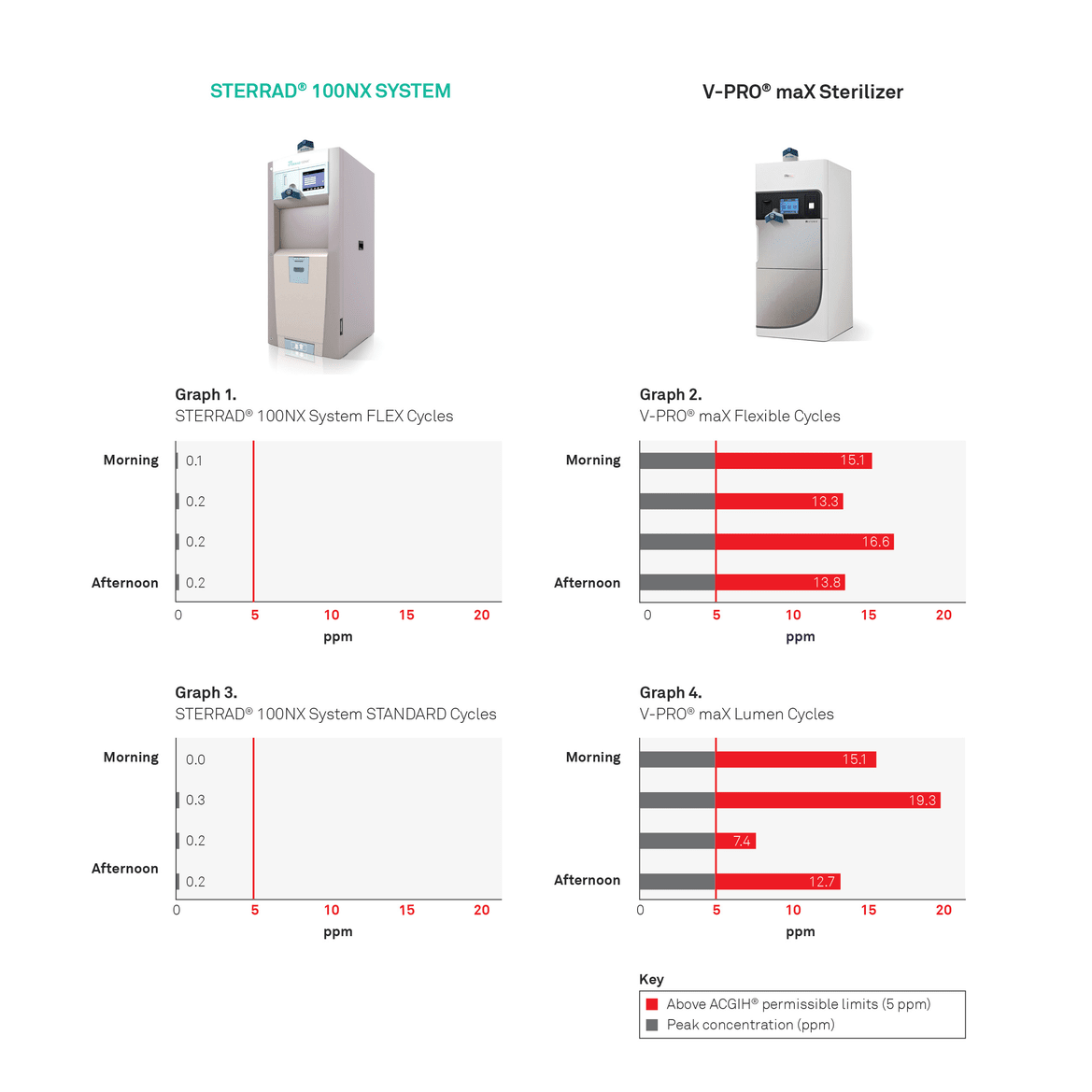
There are safety standards in place to ensure that environmental concentrations of the hydrogen peroxide remain at safe levels. The OSHA Permissible Exposure Limit (OSHA PEL) for hydrogen peroxide is currently 1 ppm,1,2 which is equal to the American Conference of Governmental Industrial Hygienists (ACGIH™) Threshold Limit Value (TLV). While this concentration limit is a time-weighted average, the ACGIH™ also has a short-term peak exposure, which states that at no time should the exposure exceed 5 ppm.3 These limits are very low, and intended to ensure worker safety in a compliant workplace.
Likelihood of Exposure: A Technological Perspective
Manufacturers design their systems to ensure environmental hydrogen peroxide exposures are kept to a minimum. Advanced Sterilization Products claims that use of a gas plasma phase in the STERRAD™ System sterilizer process dissociates unreacted hydrogen peroxide into oxygen and water, eliminating the need for aeration. Alternately, the STERIS V-PRO® sterilizers pass hydrogen peroxide through a catalytic converter where it is reduced to water and oxygen.4
In line with environmental standards regulating exposure of hydrogen peroxide, a comparison study5 was conducted to determine the differences in hydrogen peroxide emissions for both STERRAD™ Systems and STERIS V-PRO® sterilizers.