Flexible Endoscope Reprocessing: The Journey of a Dirty Endoscope
Endoscopy is a procedure to visually examine the inside of the body using an endoscope. Duodenoscope is an example of a flexible endoscope used in endoscopic retrograde cholangiopancreatography (ERCP) procedures and have received attention due to the infections associated with the improper reprocessing in recently years.1
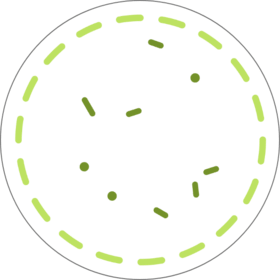
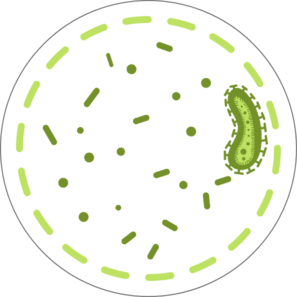
Duodenoscope are complex instruments that contain intricate working parts, and if not thoroughly cleaned and disinfected, tissue or fluid from one patient can remain when it is used on a subsequent patient.1