Sterilization of medical devices is a critical component of patient safety in healthcare settings. With the increasing complexity and delicacy of medical devices, traditional high-temperature sterilization methods are often inadequate. This challenge has driven the adoption of advanced low-temperature sterilization methods like Gas Plasma Sterilization, which ensures effective sterilization without compromising the integrity of sensitive instruments1. The shift towards low-temperature sterilization reflects the evolving needs of modern healthcare, where patient safety, device longevity, operational efficiency and operators safety are paramount.
The Evolution of Sterilization Techniques
The history of sterilization in healthcare dates back to the 19th century when steam sterilization was first introduced. While steam sterilization remains effective for many applications, its limitations are becoming more apparent as medical technology advances. High temperatures and moisture can damage delicate instruments and materials, leading to the development of low-temperature methods.
Low-temperature sterilization, including Ethylene Oxide (EtO), Paracetic Acid (PAA) and Formaldehyde (FO), has been in use for decades.1 However, these methods involve hazardous chemicals, lengthy cycle times and increased costs and resource requirements, making them less ideal in fast-paced healthcare environments. The introduction of Hydrogen Peroxide Gas Plasma Sterilization represents a significant advancement in sterilization technology, offering a safer, faster, and more effective alternative.
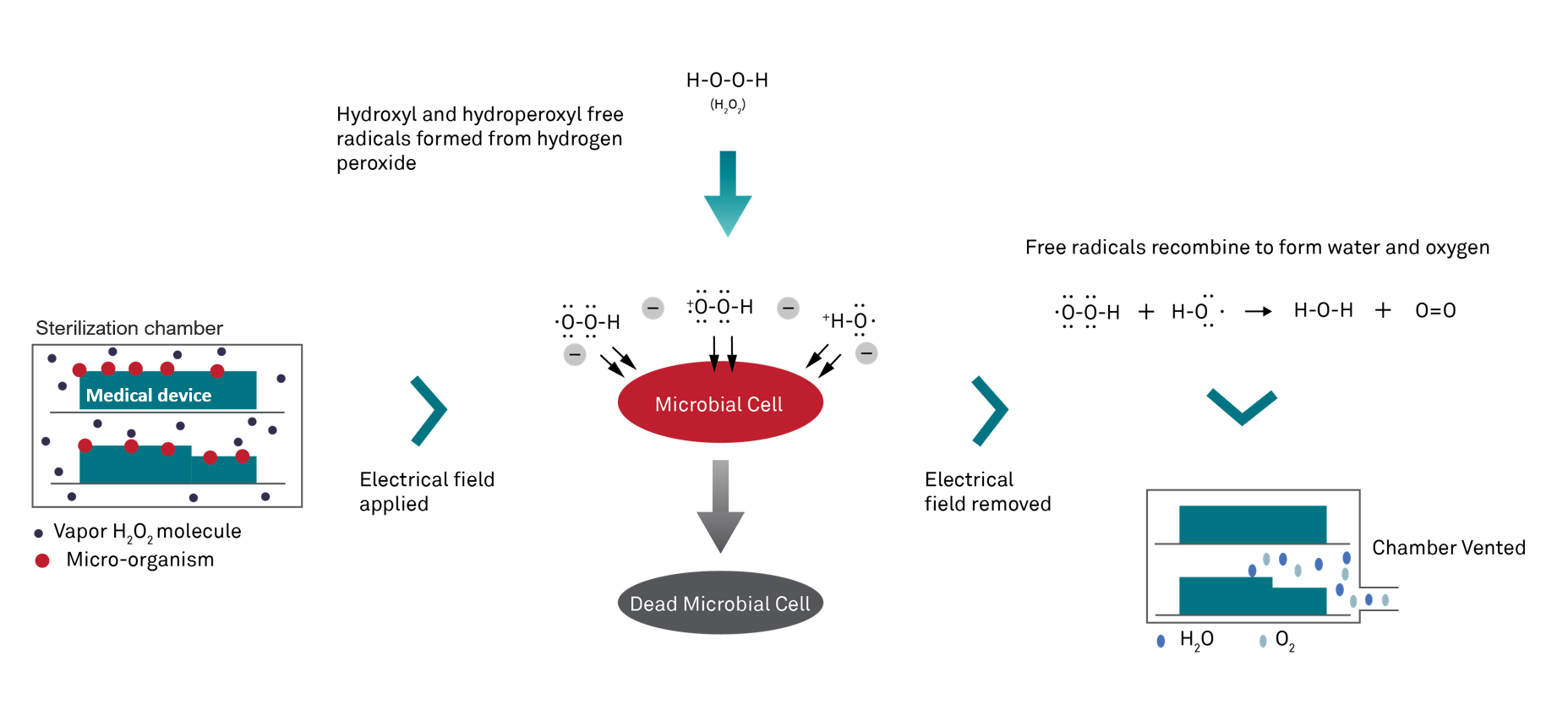
A hydrogen peroxide and water solution (59% nominal hydrogen peroxide by weight) is introduced into the sterilizer, where it undergoes further concentration.
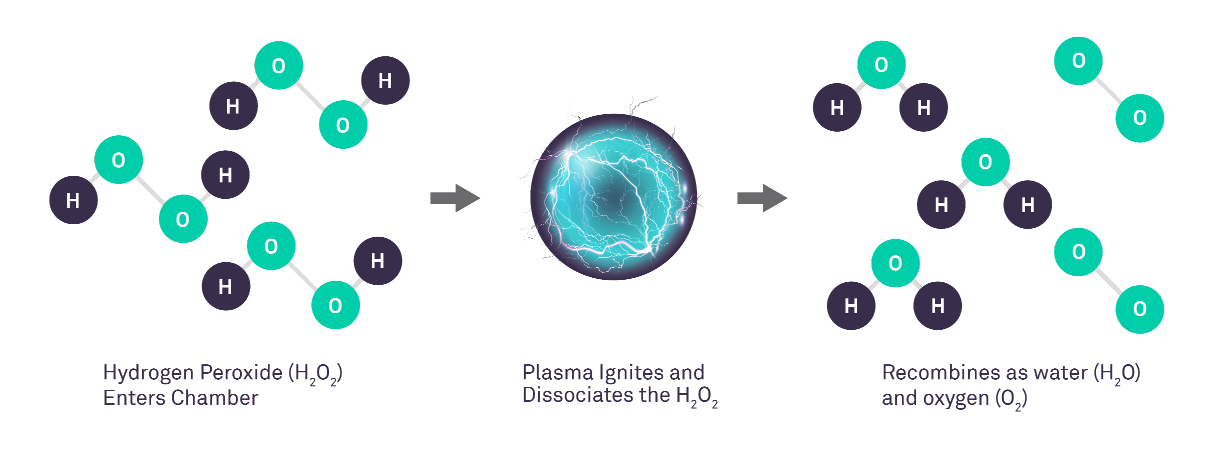
Subsequently, this concentrated hydrogen peroxide solution is vaporized into gas and transferred into the chamber, enveloping the devices and creating a biocidal environment that deactivates microorganisms through chemical interactions. This process involves the application of a strong electrical field, generating hydrogen peroxide gas plasma.
The plasma consists of highly energized species, forming a 'cloud' within the chamber. Once the electrical field is deactivated, these energized species recombine, converting the hydrogen peroxide back into harmless water and oxygen molecules.2
Compared to Ethylene Oxide (EtO) and Formaldehyde (FO), hydrogen peroxide (H2O2) presents advantages as a non-carcinogenic and non-mutagenic sterilant. Additionally, when used as gas plasma, it breaks down into non-toxic by-products, ensuring the safety of patients and staff by minimizing exposure to health risks3.
This method's safety is one of its most significant advantages. Unlike EtO and FA, which can leave toxic residues, the by-products of hydrogen peroxide gas plasma sterilization are water and oxygen, which are harmless safeguarding the safety of not only patients but also staff. Additionally, the process is conducted at low temperatures, typically between 47°C and 56°C, making it suitable for heat-sensitive and delicate instruments3.
STERRAD™ systems offer a range of benefits that make them the preferred choice for many healthcare facilities. These advantages include:
1. Enhanced Safety
Patient and staff safety are top priorities in healthcare. Traditional methods like EtO pose risks due to their toxic by-products, which can be harmful if inhaled or if residues remain on instruments. In contrast, STERRAD™ systems minimize these risks by producing only non-toxic by-products, ensuring a safer environment for both patients and healthcare workers3.
2. Efficiency and Speed
In healthcare settings, the rapid availability of sterilized instruments is crucial. STERRAD™ systems are designed to complete sterilization cycles quickly, often in less than an hour. This speed allows for a faster turnaround of instruments, reducing downtime and improving operational efficiency. The ability to quickly and effectively sterilize instruments is particularly important in emergency situations where delays can impact patient outcomes.
3. Environmental Friendliness
As the healthcare industry becomes more aware of its environmental impact, there is a growing demand for greener alternatives to traditional practices. STERRAD™ systems meet this demand by using hydrogen peroxide, which decomposes into water and oxygen. Unlike EtO and FO, which involve chemicals that contribute to environmental pollution, STERRAD™ systems have a minimal ecological footprint. The reduced energy consumption of these systems further enhances their environmental benefits, making them a more sustainable choice for healthcare facilities.
4. Versatility in Sterilizing Complex Devices
The increasing complexity of medical devices presents a significant challenge for sterilization. Instruments with intricate designs, such as endoscopes, feature narrow lumens and other difficult-to-reach areas that traditional sterilization methods struggle to penetrate. The STERRAD™ 100NX sterilizer and STERRAD NX™ sterilizer excel in this area by concentrating hydrogen peroxide, which enhances penetration into long lumens and ensures thorough sterilization. This capability is particularly important for devices used in minimally invasive procedures, where even a small amount of contamination can lead to serious complications.
5. Reduced Energy Consumption
Another key advantage of STERRAD™ systems is their lower energy consumption compared to traditional methods. The low-temperature process requires less energy to operate, contributing to lower operational costs and a reduced carbon footprint for healthcare facilities. This efficiency is particularly beneficial for large hospitals and medical centers, where sterilization is a continuous, high-volume process.
Applications of STERRAD™ Systems in Modern Healthcare
STERRAD™ systems are versatile and can be used to sterilize a wide range of medical devices, including:
1. Surgical Instruments
Surgical instruments, which often feature complex designs and are made from materials sensitive to heat, require meticulous sterilization. STERRAD™ systems ensure that these instruments are thoroughly sterilized without compromising their structural integrity or functionality. The ability to quickly sterilize surgical instruments is crucial in operating rooms, where instrument availability can directly impact surgical schedules and patient care.
2. Endoscopes
Endoscopes are vital tools in modern medicine, used in a variety of diagnostic and therapeutic procedures. However, their long, narrow channels present a significant challenge for sterilization. STERRAD™ systems’ ability to effectively sterilize endoscopes’ difficult-to-reach areas makes them ideal, ensuring that every part of the instrument is free of harmful microorganisms.
3. Delicate Devices
Many modern medical devices are made from materials that cannot withstand the high temperatures of traditional sterilization methods. These include plastics, rubber, and certain polymers. STERRAD™ systems' low-temperature process makes them ideal for sterilizing these heat-sensitive devices without causing any damage, thereby extending the lifespan of these costly and delicate instruments.
The Science Behind STERRAD™ Effectiveness
The effectiveness of STERRAD™ NX systems is rooted in their advanced technology. The plasma generated during the process is made of highly energized species. Once the electrical field is deactivated, these energized species recombine, converting the hydrogen peroxide into harmless water and oxygen molecules.2
Moreover, the low-temperature nature of the process ensures that even heat-sensitive materials, such as certain plastics and rubber components, can be sterilized without damage. This broadens the range of instruments that can be safely and effectively sterilized using STERRAD™ systems, making them suitable for use across various medical specialties.
The Future of Sterilization: Innovation and Beyond
As healthcare continues to advance, so will the technologies used in sterilization. The STERRAD™ system represents a significant step forward in this field, but the future holds even more potential for innovation. Future advancements may focus on further improving efficiency, reducing environmental impact, and enhancing the ability to sterilize an even wider range of materials and devices.
Conclusion: Embracing Advanced Sterilization Technologies
In conclusion, the adoption of advanced sterilization technologies like STERRAD™ systems is essential for modern healthcare. These systems provide a safe, efficient, and environmentally friendly alternative to traditional methods, ensuring that medical instruments are thoroughly sterilized and ready for use in the shortest possible time. By embracing such innovations, healthcare facilities can enhance patient safety, improve operational efficiency, and contribute to a more sustainable future.
References
CDC Infection Control Low-Temperature Sterilization Technologies
Ethylene Oxide and Hydrogen Peroxide Gas Plasma Sterilization: Precautionary Practices in U.S. Hospitals. James M. Boiano, MS and Andrea L. Steege, PhD
