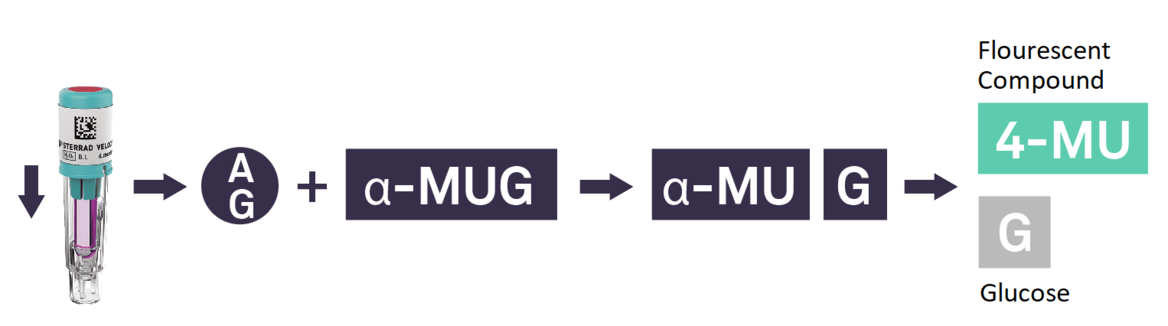The Evolution of Biological Indicator Systems
A decade ago, biological indicator (BIs) used a visual readout method to determine if a sterilization cycle successfully inactivated microorganisms. Because these antiquated BIs took one to seven days for growth readout results, they were an untimely indicator of potential catastrophic sterilization failures.
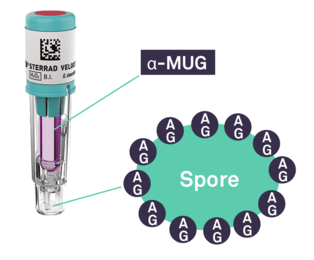
In recent years, the time to read for BIs drastically improved with the introduction of fluorescence readout. Today, the fastest time to results in the market is the STERRAD VELOCITY™ System with a 15-minute* readout.