Vaporized Hydrogen Peroxide Gas Plasma Technology
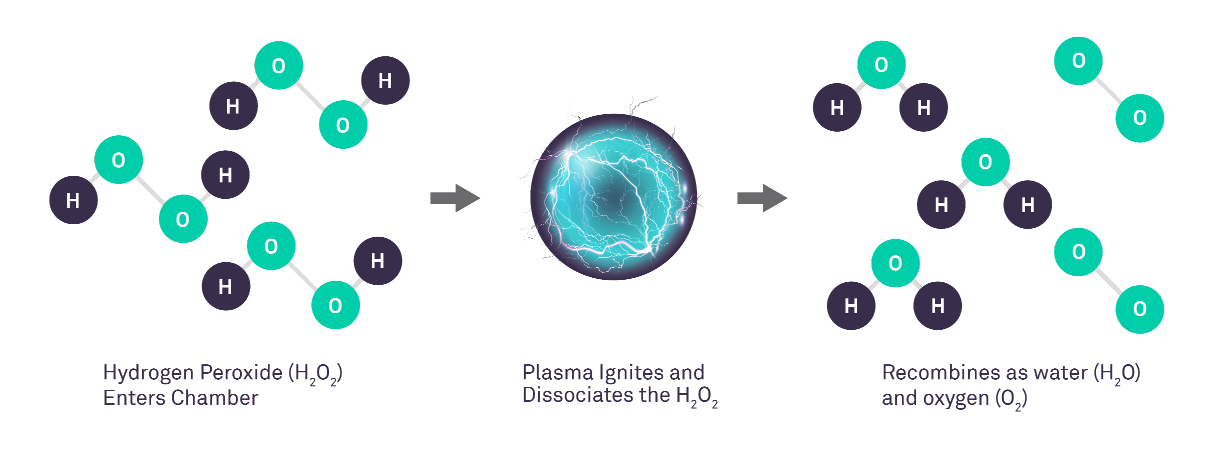
STERRAD™ Sterilization Systems utilize a combination of hydrogen peroxide (H2 O2) and low-temperature gas plasma to rapidly and effectively sterilize validated medical devices and materials, without leaving toxic residue. This approach offers safety advantages over alternative reprocessing modalities such as ethylene oxide (EtO) and other H2 O2 systems by reducing H2 O2 vapor emissions, as described below.