Hydrogen Peroxide Monitoring
Hydrogen peroxide monitoring in the STERRAD NX™ Sterilizer and STERRAD™ 100NX Sterilizer ensures a maintained concentration of hydrogen peroxide throughout the exposure period, to guarantee effective microbicidal activity. The STERRAD NX™ Sterilizer and STERRAD™ 100NX Sterilizer both feature control systems with fully integrated hydrogen peroxide monitors, which directly measure the chamber sterilant concentration, as standard. The hydrogen peroxide monitor provides data to the process controller, which also considers the acceptable cycle limits, as determined by statistical analysis of microbiological efficacy testing, to ascertain the acceptability of each cycle and ensure a minimum sterility assurance level (SAL) of 10-6.
STERRAD™ System’s in-built, continuous hydrogen peroxide monitor is comprised of an ultraviolet (UV) light source and detector, which records fluctuating interactions between hydrogen peroxide vapour and UV light intensity. When hydrogen peroxide declines to a concentration below a defined threshold for sterilization the UV light intensity changes, signalling for the sterilization cycle to be halted.
Gas Plasma
STERRAD™ Systems, also referred to as Vaporised Hydrogen Peroxide Gas Plasma Sterilization or Low Temperature Sterilization, directly remove residual hydrogen peroxidefrom the chamber and instruments using an exclusive gas plasma technology. As hydrogen peroxide diffuses through the chamber and surrounds the loaded instrument, the low-temperature gas plasma is ignited via the application of an electric field. As a result of the plasma, the hydrogen peroxide vapour splits into free radicals. When the electric field through the plasma is terminated, the free radicals lose their high-energy state and recombine as oxygen and water vapour.
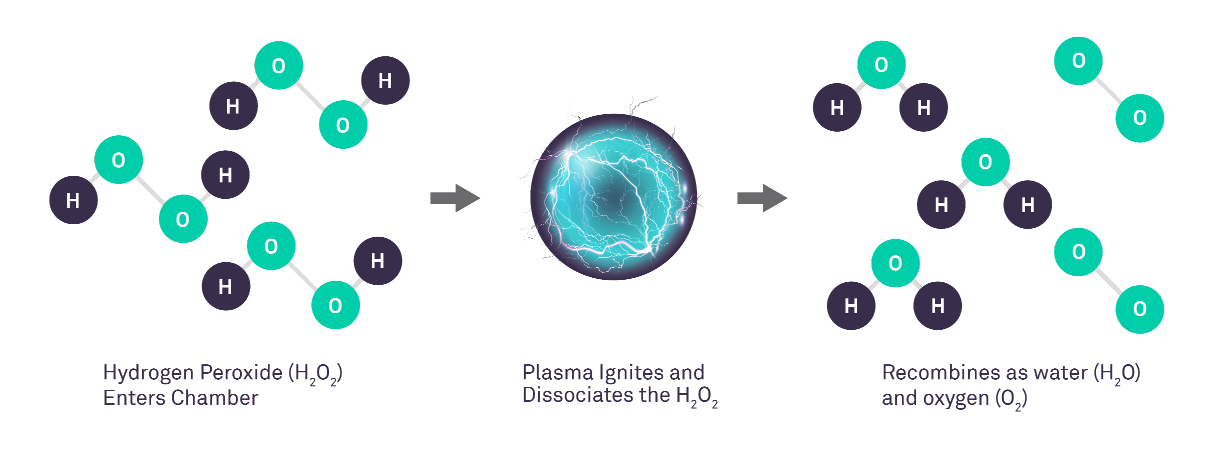
Gas Plasma Process
STERRAD™ Systems emit significantly less hydrogen peroxide than STERIS® VPRO-Sterilizers, minimising the hydrogen peroxide exposure that healthcare workers receive when opening the sterilizer door VPRO-Sterilizers.
In a comparative study of continuous hydrogen peroxide emission monitoring2, V-PRO® Sterilizer emissions were 67 times higher than those of STERRAD™ Systems upon opening the sterilizer door after a cycle. This highlights that STERRAD™ Systems, which use a gas plasma phase to dissociate hydrogen peroxide during the sterilization cycle, are more effective in reducing hydrogen peroxide than the STERIS® V-PRO® maX, which only passes H2O2 through a catalytic converter.