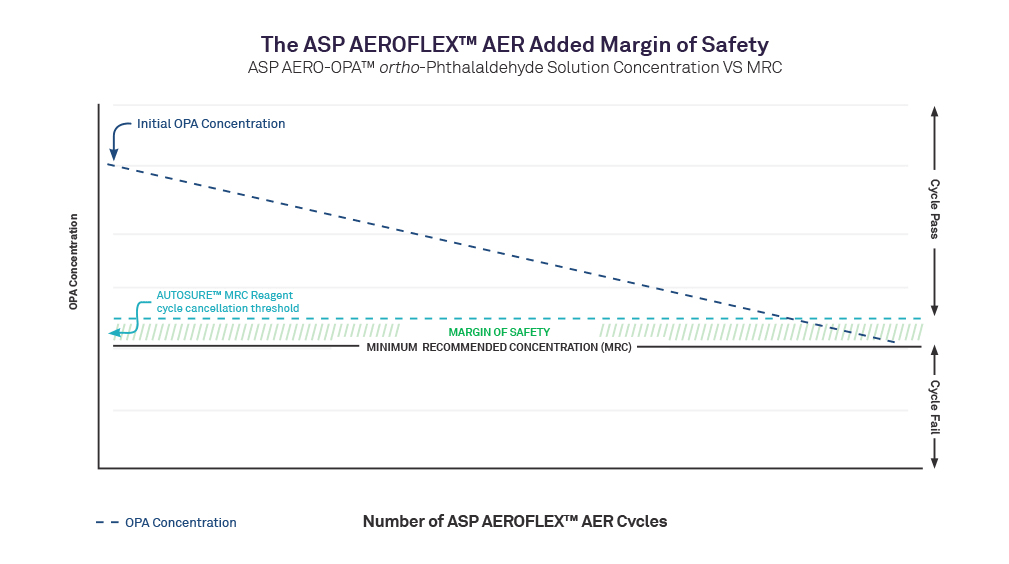
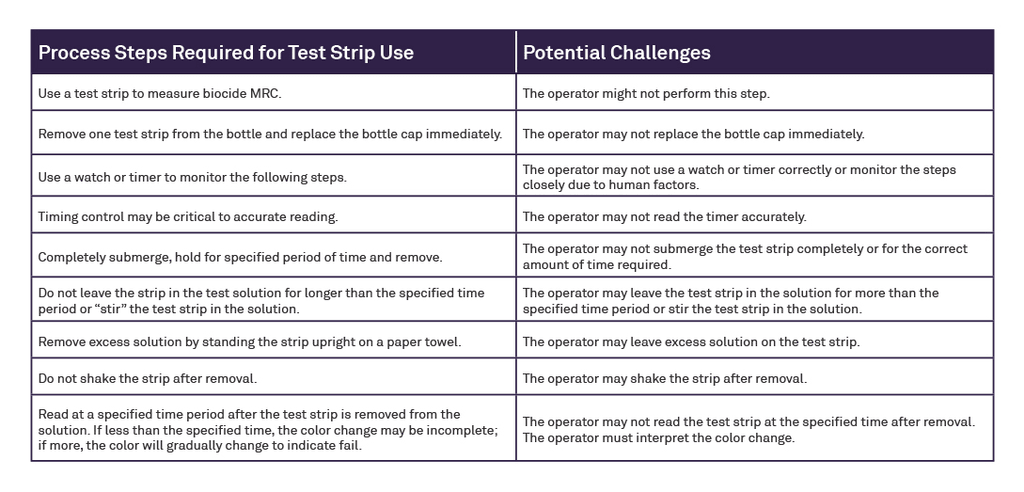
Abstract
Reprocessing flexible endoscopes in today’s fast-paced clinical environment demands consistent, effective high-level disinfection (HLD) using automated endoscope reprocessors (AERs). Yet, despite advancements in technology over the last decade, AER operators are still required to manually test biocide minimum recommended concentration (MRC) levels during every cycle using a test strip. With the introduction of the ASP AEROFLEX™ AER with AUTOSURE™ MRC Monitor, automatic minimum recommended concentration testing for every cycle is a standard feature, designed to increase compliance and patient safety and reduce the risk of healthcare-acquired infections during endoscopy procedures. It is a novel automated concentration measurement system grounded in mature chemistry and colorimetry; a summary technical explanation is presented in this document.
Endoscopy centers have become laser-focused on keeping procedure rooms fully scheduled to maximize the use of resources and patient throughput. This has a ripple effect throughout the chain of support services which enable supplies, capital equipment and instruments to be ready and available when the patient and physician are scheduled for an endoscopy procedure. This is in no small part due to the rapid rise in endoscopy procedure volumes over the past several years3. According to a study published by iData in 2019, there were 31 million GI endoscopy procedures performed in the United States alone in 20184, and there is every reason to expect continued procedure growth over the next 2-3 years.
This rise in endoscope use places strain on an already challenged reprocessing environment, where pressure to increase volumes of scopes prepared for the next procedure invites compromises in manual cleaning and disinfection procedures, which has been well documented in recent peer-reviewed research.5
To meet the increased procedure demand, endoscopy centers have responded with investment in tools and staff to manage the expanding workload, and the AER is playing a tireless role in ensuring endoscopes are available and properly disinfected as needed.