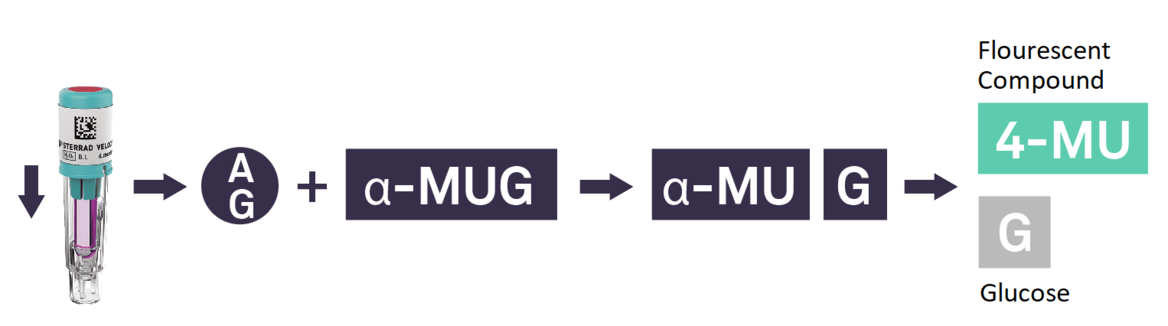The Evolution of Biological Indicator Systems
A decade ago, biological indicators (BIs) used a visual readout method to determine if a sterilization cycle successfully inactivated microorganisms. Because these antiquated BIs took one to seven days for growth readout results, they were an untimely indicator of potential sterilization failures.
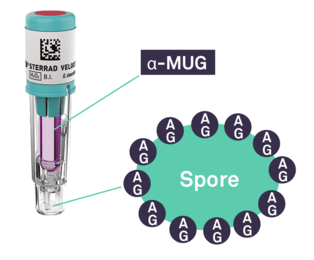
In recent years, the time to read for BIs drastically improved with the introduction of fluorescence readout. Today, the STERRAD VELOCITY™ System* offers the quickest turnaround time for a Biological Indicator/Process Challenge Device (BI/PCD) with a readout time of 15 minutes**.